DocControl is a HIPAA Compliant Document Management Software providing electronic document management in an efficient and cost effective manner.
Find out the list of Product features on left.

This ensures that only the current version of a standard operating procedure (SOP) is viewable to a user. Revision control may be coupled with one or more Document Approvers in environments where specific approvals prior to implementation are required.
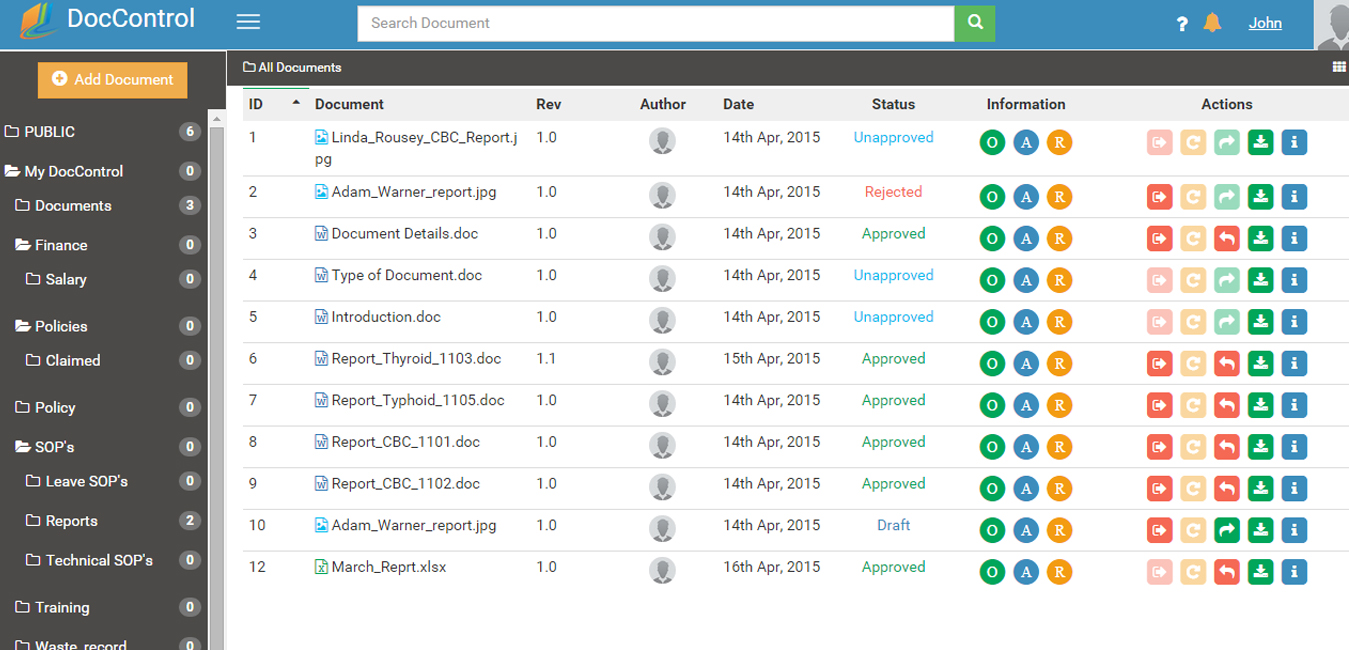
DocControl provides a secure, time-stamped audit trail exceeding 21 CFR part 11 requirements. The audit trail captures all document processes, from the original author to any changes made to the document. Naturally, all revisions are kept and available for review or auditing purposes.
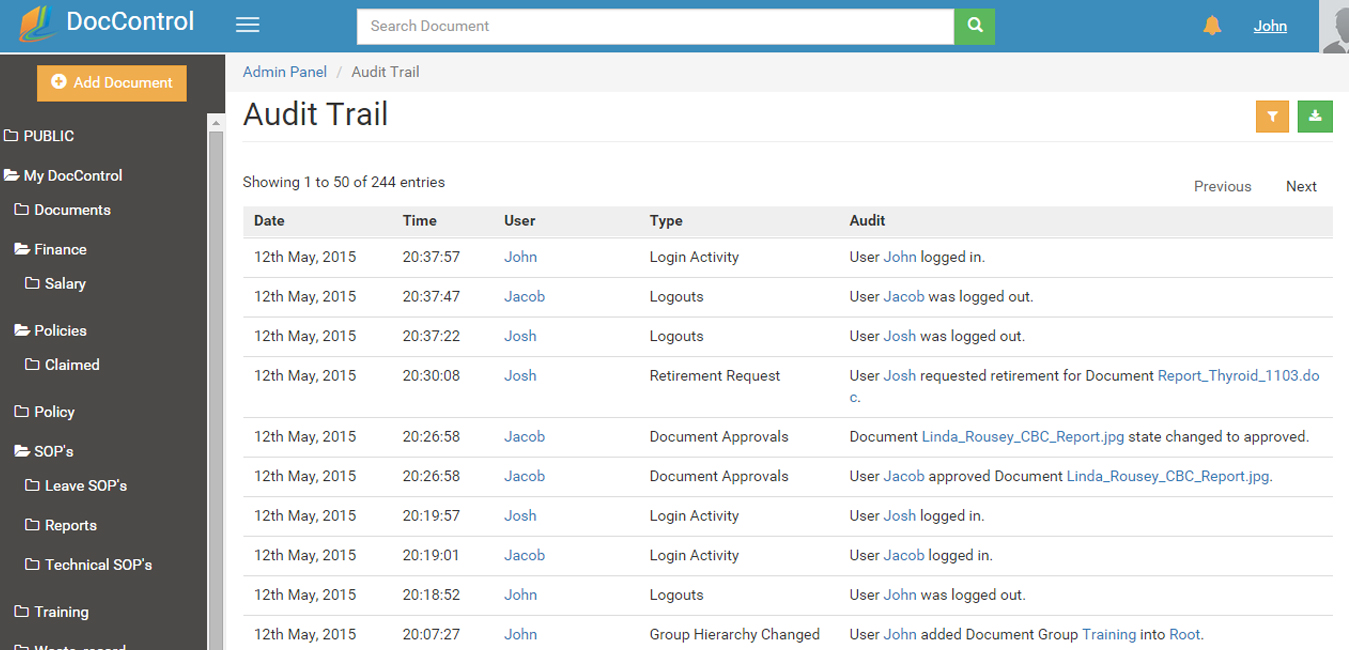
DocControl has powerful search functions allowing you to identify documents by group location, by status or history. A document can be either in the Draft, Review, Approved or Retired state. The revision or approval history of electronic documents can also be reviewed using the history feature.
Electronic documents are signed prior to approval and publishing to the Document tree. Electronic signatures including name, date, time, and meaning of electronic signature and are appended automatically to each document.
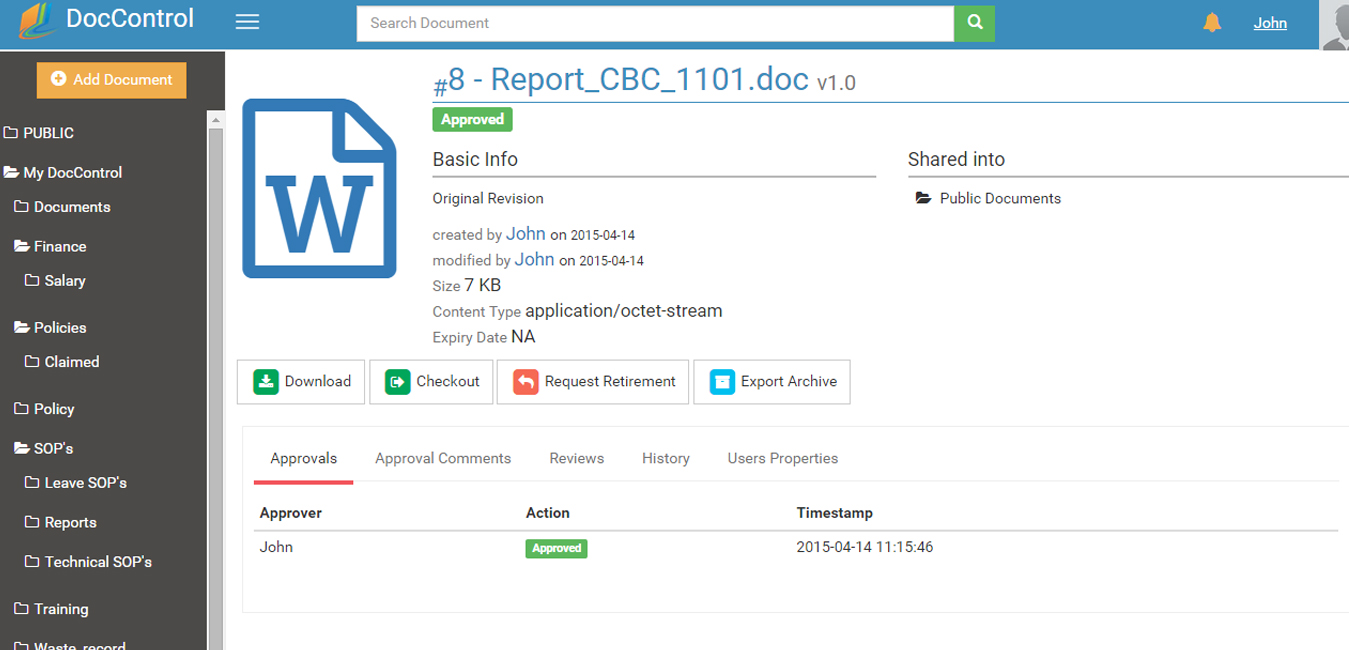
DocControl is Web-based so it can connect all employees involved in document and quality control from virtually anywhere. DocControl provides a centralized repository for easy access, search, and retrieval of electronic documents by all authorized users.
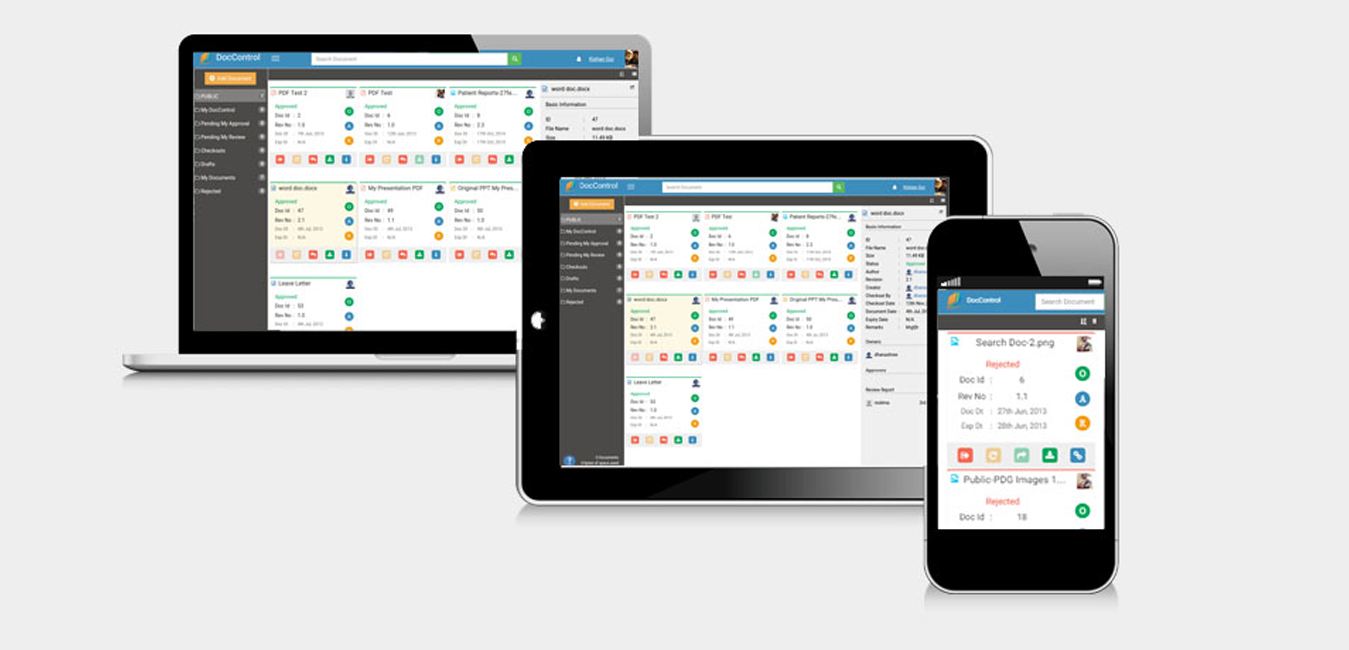
DocControl provides pre-defined template section from which a new document can be derived.
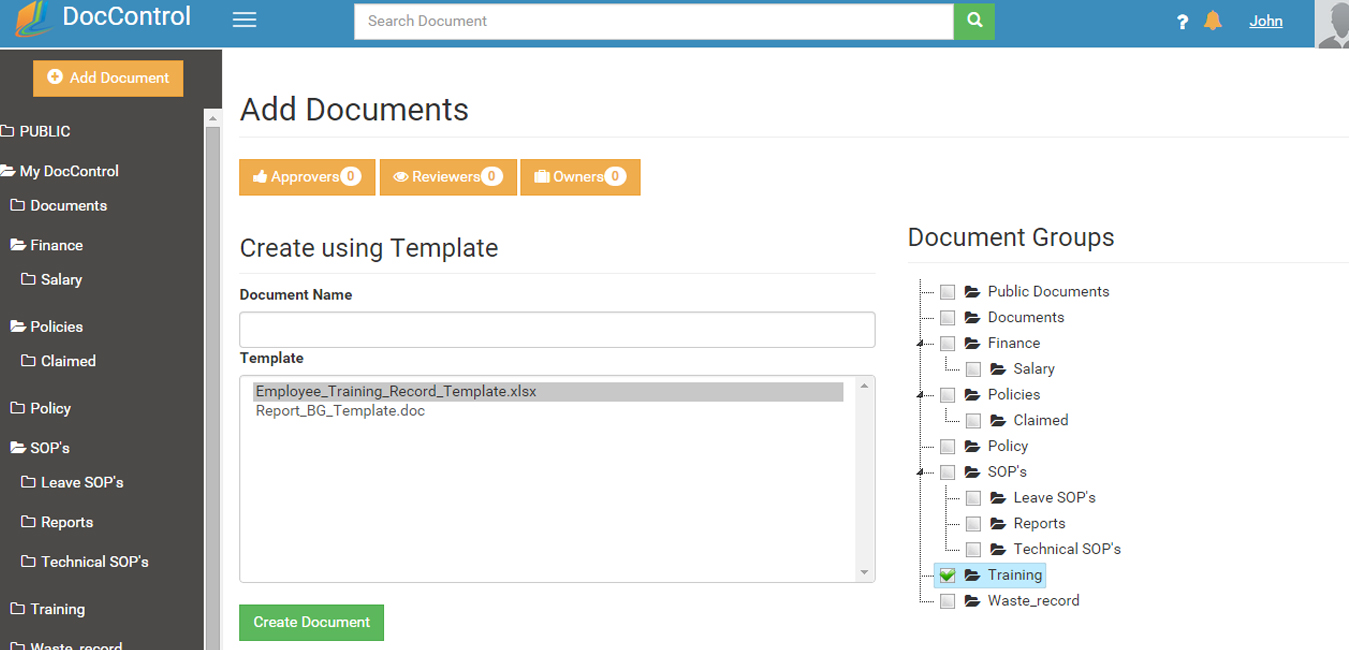
DocControl provides a standardized Google-like search window that enables authorized users to search the entire system quickly and efficiently
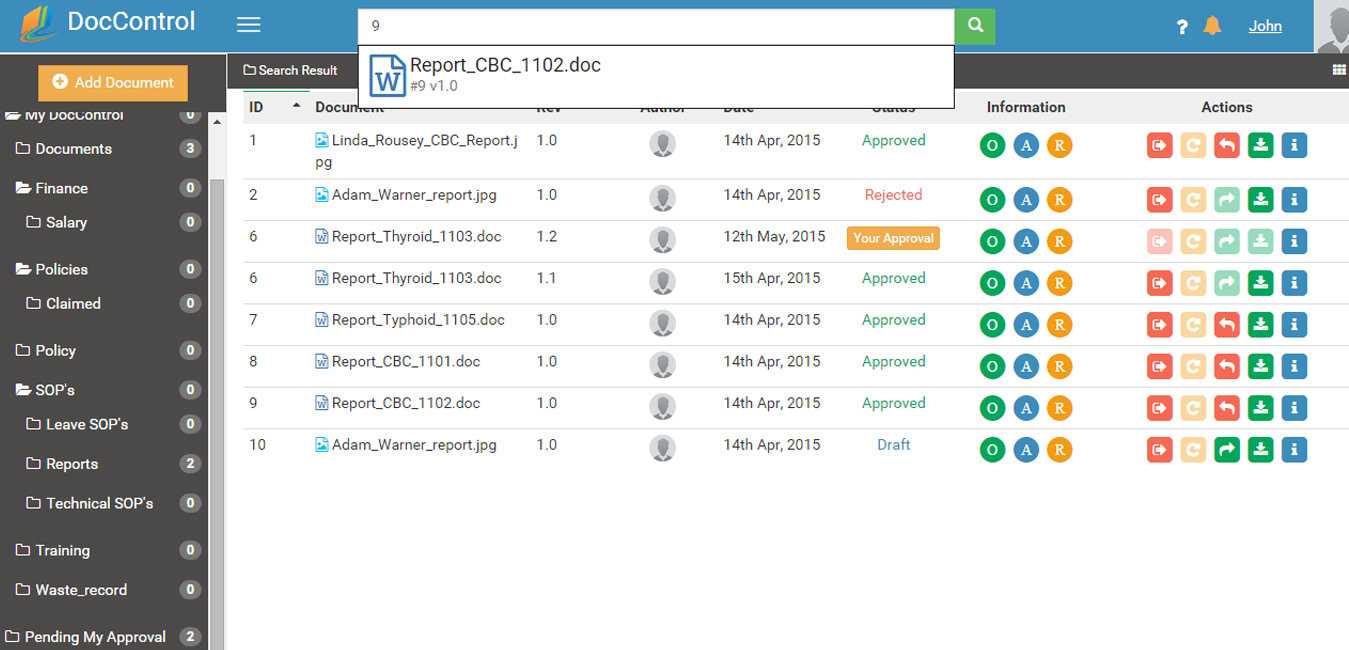
Documents are converted automatically to PDF at approval. PDFs can be customized to include a watermark, header page, and footer. Security features include PDF encryption, cut, copy, paste, and password protection for viewing or printing.

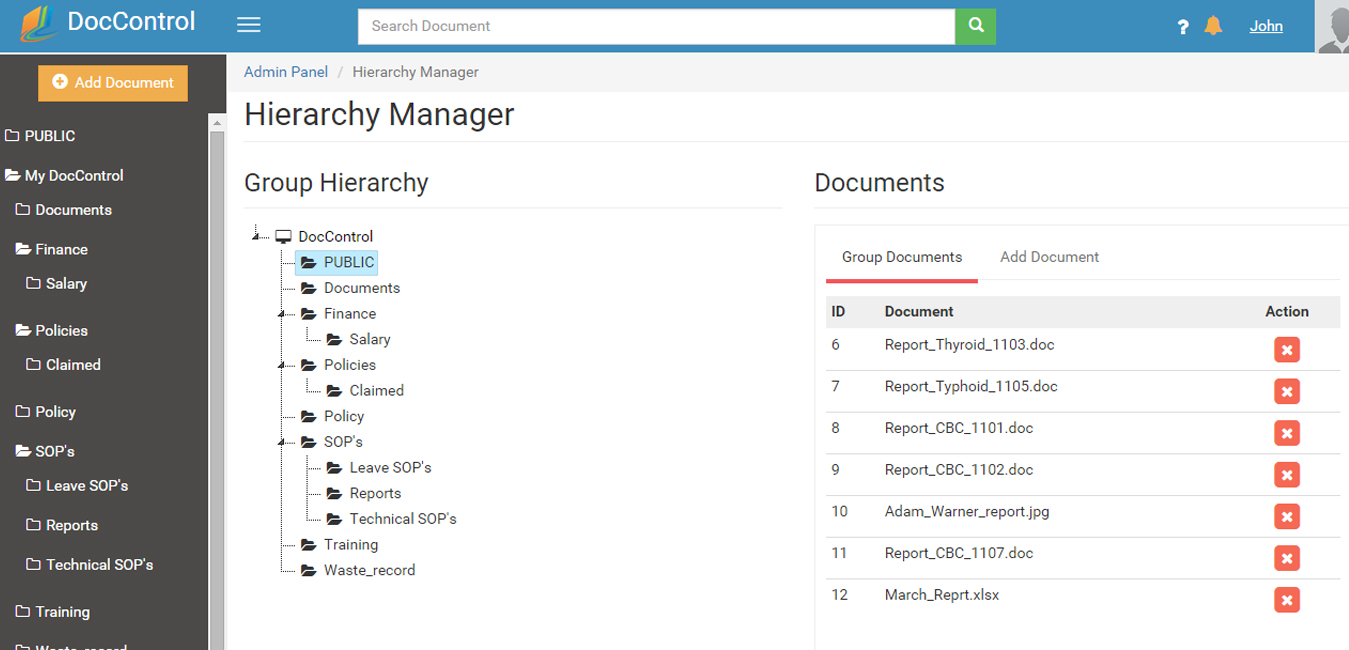
Granular access rights and flexible Document groups, allows you to create a document structure and user access that is perfect for your organization. Document Groups have a a familiar document tree structure, with granular accesses permissions. Documents take the properties of the document group they are placed into (unless overridden). Documents can be a member of multiple groups.
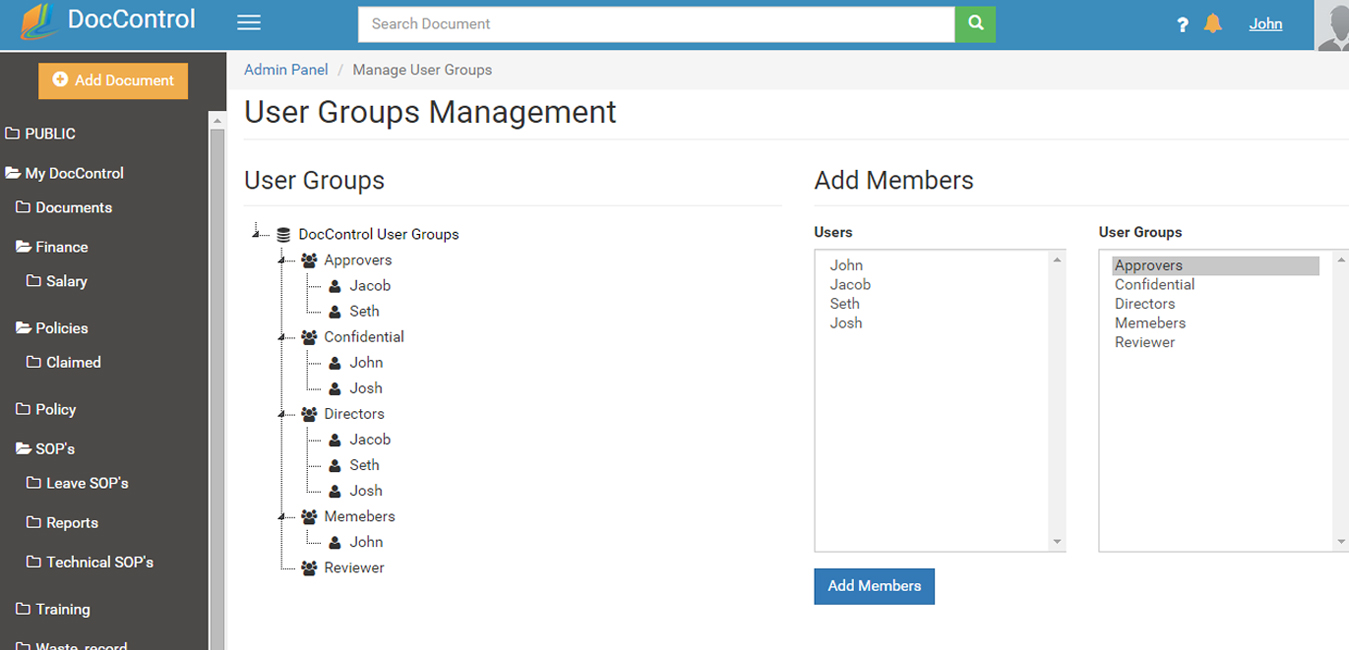
Users can be grouped into user groups User providing an intuitive way of organizing the members of your project. Groups members inherit the same permissions and privileges making it easy to add or remove members.
Document reviews are scheduled and automatic notifications sent to applicable personnel to perform them. Should reviews not be performed within the appropriate timeframe DocControl provides the ability to escalate the action to ensure appropriate follow-up
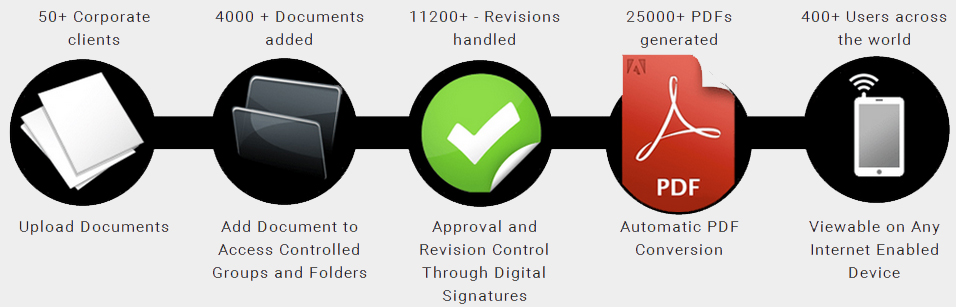
DocControl provides effective pharmaceutical document control for the structured management of regulated content across Research and Development, Operations, and Marketing at biotechnology and life sciences companies.

Document control is essential in the success of quality management. Quality documentation like CAPAs, SOPs, non-conformance reports, employee training, and work instructions are managed within a document control repository that provides for version tracking, approvals, notifications, policy administration, and more.
DocControl is a Web based Document Control and Management System. It has intuitive and Responsive interface which makes it accessible on any device. It allows multiple users to work in a team, providing services such as massive storage, versioning of documents, electronic signatures and automatic PDF conversion. DocControl is HIPPA and 21 CFR part 11 compliant product that makes it more secure than any other document management system. Any type of documents can be added into DocControl and it will automatically get converted into PDF. It has the awesome workflow for document revision and every time the document revised its version number gets updated automatically. DocControl is suitable for small and medium size business that provide security to their documents and works in a team. DocControl has more than 50 Corporate Clients, 5000 + documents added into the system and more than 30,000 PDF generated.